Spinal Cord Stimulation
What is the purpose of this procedure?
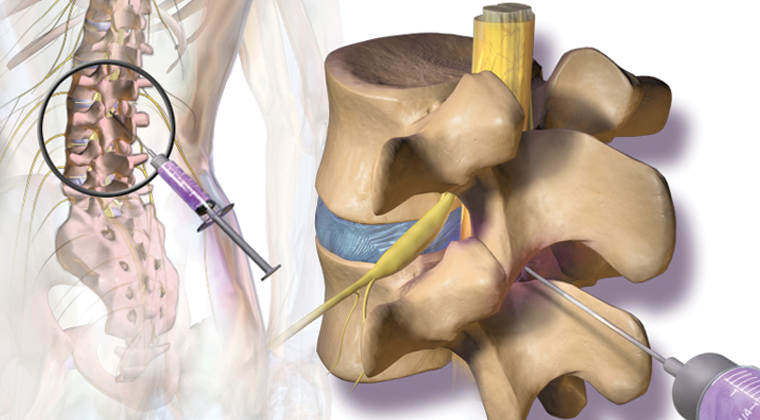
Spinal cord stimulation (SCS) is an implantable pain relief therapy. The surgeon implants a device into the body that sends a low electric current to your spinal cord to block pain signals traveling to your brain. SCS has been FDA approved to relieve chronic pain from nerve damage in the trunk, arms, or legs since 1989. The most common situations it is used for are failed back surgery, complex regional pain syndrome, and peripheral neuropathic pain caused by nerve damage beyond the spine or brain. The permanent device used in the therapy is similar in appearance to a pacemaker. It consists of an impulse generator, battery and leads to carry the electrical signal to the target nerves. When considering if SCS may be helpful for you, it is important to have a thorough assessment with your doctor. Following this a test period called a "trial" is arranged. During the trial a temporary device is used to give you an idea of what it would be like to have a stimulator. Trials typically last seven days. At the end of the trial the temporary leads are removed. If the trial is successful, a permanent system can be implanted through a minimally invasive surgery.
What are the risks associated with this procedure?
The risks are infrequent. They include:
• Allergic reaction to medication
• Nerve damage (spinal cord and nerve roots)
• Bleeding and bruising at the injection site
• Pain at the injection site or during the procedure
• Infection
• Puncture of the sac surrounding the spinal cord (dura mater)
• Spinal headache
• No improvement or worsening of your pain in some cases
A common complication is movement of the leads during the trial. This is called lead migration. If the leads move during the trial your painful areas may not be covered. This makes it hard to determine whether or not it would work for you.
How should I prepare for the procedure?
• You must have a driver with you at the time of check-in and check-out. Your driver must accompany you to the clinic for your procedure. You can be discharged only to the care of a responsible adult driver 18 years of age or older.
• Since you will be receiving sedation, it is important that you do not eat within 6 hours before the procedure. Small amounts of clear liquids are ok up to 2 hours before the procedure. If you have diabetes, discuss an eating and medication schedule with your doctor.
• You may need to stop taking certain medications several days before the procedure. Please remind the doctor of all prescription and over-the-counter medications you take, including herbal and vitamin supplements. The doctor will tell you if and when you need to discontinue the medications.
• It is very important to tell the doctor if you have asthma or had an allergic reaction to the injected dye for a previous radiology exam (CT scan, angiogram, etc.). An allergic reaction has symptoms such as hives, itchiness, difficulty breathing, or any treatment which required hospital stay.
• Tell the doctor if you develop a cold, fever, or flu symptoms before your scheduled appointment, or if you have started taking antibiotics for an infection.
What will happen during the procedure?
The procedure is performed on an outpatient basis in a special procedure room equipped with a fluoroscope (x-ray). In the pre procedure area the nurse or doctor will place an IV line. This is used for fluids and sedation.
- When you get to the procedure room for your safety and comfort you will be connected to monitoring equipment (EKG monitor, blood pressure cuff, and blood oxygen monitoring device), and positioned on your stomach.
- Your back is cleansed with an antiseptic soap after which the doctor injects numbing medicine into your skin. This will cause a burning sensation for a few seconds.
- The doctor then carefully directs a needle with help of the fluoroscope (x-ray) to the epidural space.
- Once positioned correctly, a thin lead is passed through the needle. The lead is advanced until the small contacts on the end of the needle are over the target location.
- The leads are connected to the impulse generator then turned on. You will feel a tingling sensation called a paresthesia. The device representative will ask you several questions such as where you feel it and how strong it is.
- When the device is in the correct location it will be secured in place.
What should I do after the procedure?
• After the procedure you will be taken to the recovery area where you will spend about 30 minutes. The device representative will program the stimulator and teach you how to use it.
• After you are finished you will go to Radiology to receive an x-ray before going home. You will return in 1 week to have the trial leads removed and to evaluate the trial.
Content supplied by:
Ronald Wasserman, M.D.
1.29.2020
Disclaimer: This document contains information and/or instructional materials developed by Michigan Medicine for the typical patient with your condition. It may include links to online content that was not created by Michigan Medicine and for which Michigan Medicine does not assume responsibility. It does not replace medical advice from your healthcare provider because your experience may differ from that of the typical patient. Talk to your healthcare provider if you have any questions about this document, your condition or your treatment plan. Patient Education by Michigan Medicine is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported (CC BY-NC-SA 3.0) License. Last Revised 06/2018.